Immunotherapy is the treatment of a disease, like cancer, by activating or suppressing the patient’s immune system, thereby identifying and destroying cancer cells. Chimeric antigen receptor T-cell therapy (or CAR-T therapy) is the latest development in this field with tremendous potential to revolutionize cancer therapy.
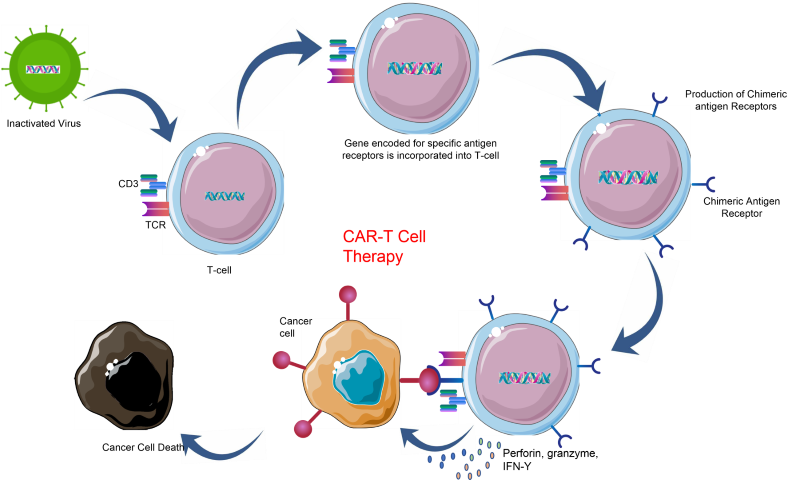
Chimeric antigen receptor T-cells (CAR-T cells) are specialized T-cells that have been genetically engineered to produce an artificial T-cell receptor designed for use as an immunotherapeutic agent. This allows the CAR-T cell to target and selectively kill cancer cells when administered to a patient. T-cells are harvested from a patient (autologous) or taken from a healthy donor (allogeneic) and genetically altered by the introduction of a retro- or lentiviral construct expressing a specific T-cell receptor which programs the modified T- cells to identify and target an antigen present exclusively on the surface of tumour cells. In the weeks following the introduction of the chimeric T-cell into the patient, it behaves as a normal T-cell but with the ability to recognize and eliminate cancer cells, which the patient’s unaltered T-cell was unable to do.
CAR T-cells have been the focus of multiple clinical trials recently, with promising results that demonstrate their efficacy and specificity, leading to less harmful side effects when compared to radio- or chemotherapy. CAR-T cells also have the advantage of being retained in the patient to develop into long-lived memory T-cells. This can prevent the recurrence of the same cancer in the patient, if there is a relapse, the memory CAR T-cells are reactivated immediately to eliminate the new cancer cells swiftly. As CAR-T therapy is completely different from existing cancer therapies, the monitoring and assessment of its effects also require novel methodology. CAR-T cells require characterization or ‘immunophenotyping’ by Flow Cytometry, cell-based assays, and qPCR/RT-PCR. Another parameter that requires careful monitoring during CAR-T therapy is patient health. This usually involves the measurement of cytokines and antibodies produced by the patient’s immune system in response to the treatment, by ELISA, MSD, Luminex, cell-based proliferation assays, etc., both during treatment and in the months and years following the treatment.
ImmunitasBio is uniquely placed to provide these assessments by applying highly specialized scientific capabilities to cutting-edge platforms resulting in rapid, high-quality, reproducible results in the following areas:
Cellular immunogenicity testing by Flow Cytometry