For Vaccines
Owing to the serious and far-reaching impacts of emerging diseases like COVID-19, Dengue, Zika, and Ebola and the resurgence of old diseases like measles, malaria, and tuberculosis, vaccine development has become one of the most critical and rapidly accelerating areas in pharmaceuticals and biotechnology. The importance of vaccines in public health on a global scale is now firmly established and subject to much attention from society and the science fraternity as a variety of new-generation vaccines are being researched and developed, especially for new and persisting diseases with no known cure.

For Immuno-Oncology
Immunosurveillance is the process by which the cells of the immune system, especially lymphocytes, look for, recognize and eliminate pre-cancerous and cancerous cells in our body. This forms the first step of the general process of immunoediting, which involves the transformation of cancerous cells to escape immunosurveillance giving rise to malignancy. The study of these processes, initiated by Burnet and Thomas in 1957, and supported by advances in the understanding of both antibody-mediated and cell-mediated immunity and the intricate complexity of the immune system, have since aided in the development of various cancer immunotherapies. These therapies aim at activating the immune system to combat and eliminate cancerous cells in the body.

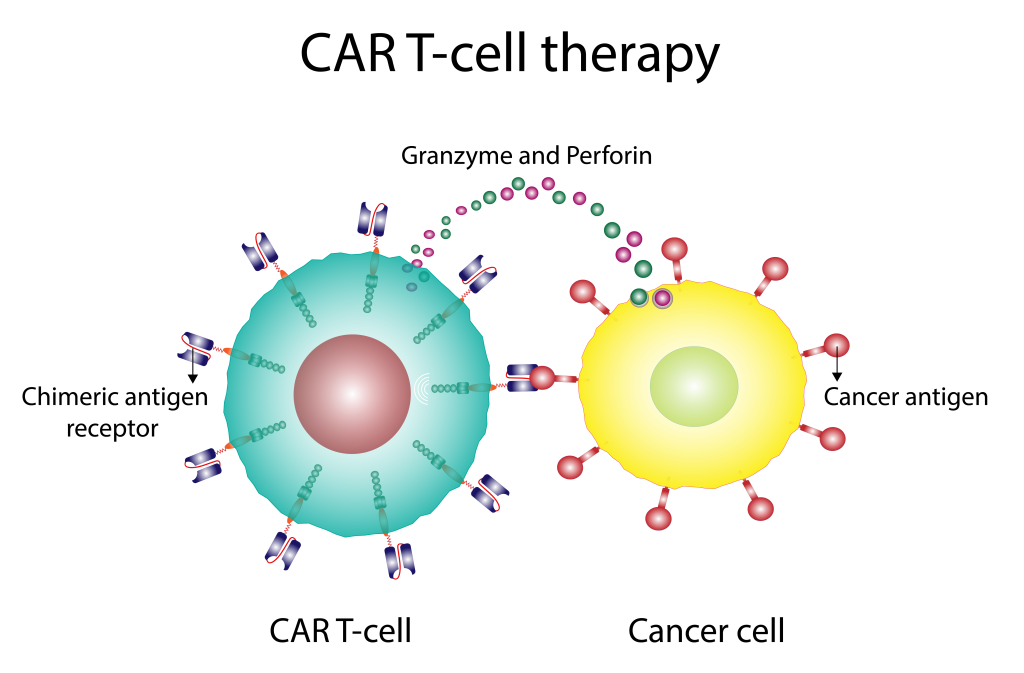
For CAR-T cell Therapy
Immunotherapy is the treatment of a disease, like cancer, by activating or suppressing the patient’s immune system, thereby identifying and destroying cancer cells. Chimeric antigen receptor T-cell therapy (or CAR T therapy) is the latest development in this field with tremendous potential to revolutionize cancer therapy.
For Allergy
Exposure to Allergens in the workplace or at home can lead to sensitization and exacerbation of allergy symptoms and asthma. Allergen avoidance is one of the primary measures advised for the prevention of allergy symptoms and asthma. In this regard, numerous products are under development by pharmaceutical, biotechnology, and FMCG companies that either prevent individuals from exposure to allergens or reduce the allergens in the environment they inhabit.

For Microbiological-testing
Regulatory requirements for medical devices (including disinfectants and antiseptics) as established by the Medical Devices Directive (western market) make it imperative for all such devices to be manufactured through safe and verifiable processes that comply with health standards and environmental mandates. Unless a product follows the directive completely, it cannot be introduced into the market.

For Assay Development & Technology Transfer
We specialize in designing and executing the development of novel immunoassays for drugs and biomarkers. This includes identifying the best sources for proprietary reagents and consumables required for bioanalytical assay development, subcontracting parts of the assay development to third parties, and testing at all intermediate stages.


